By: Geeq on Apr 7, 2021
Billions of dollars are wasted each year in the pharmaceutical supply chain. Geeq’s technology provides a low-cost way to track and document supply chain data in ways that preserve privacy, yet can be shared readily should their accuracy need to be proven to other parties.
When you drop into your local pharmacy for your medication — whether it is a box of vitamins or a drug prescribed by a physician — you probably do not question the product’s quality or worry whether it will be in stock. But the fact that your medicine is waiting for you on the shelf is an organisational miracle and each box has a long journey behind it.
In most cases, this “invisible story” culminates in a happy ending: a patient who receives the right treatment. However, the journey from the manufacturing facility is long and perilous. According to the Corporate Finance Institute, a supply chain is the system used to produce and deliver a product, from sourcing the raw materials right up to to the final delivery to the end customer.
The supply chain of medicine is especially complex, however. Drugs are not only very delicate products that require special care, but delivery involves multiple stages, stakeholders, and border-crossings in a series of carefully orchestrated and regulated steps with precise timing. In such a complex endeavor, many things can go wrong and when they do, it is crucial to have a transparent record of events in order to learn from mistakes and continuously improve supply chain processes.
The journey begins
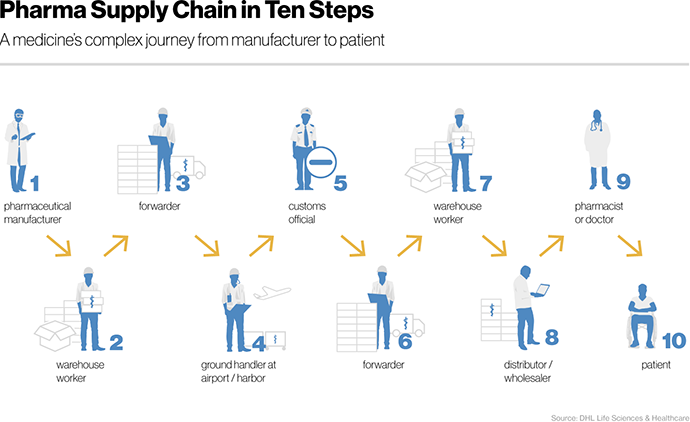
To illustrate some of the most daunting challenges, let’s return to the journey of our hypothetical medicine from factory to pharmacy. Logistics, which concerns the physical delivery of medicines, is only a part of the entire pharmaceutical supply chain. Nevertheless, a Novartis source asserts that it can amount to nearly 40% of total operating expenses. According to the same source, a medicine usually passes through at least “ten hands” before a doctor or patient receives it.
The first two of these touchpoints are the manufacturer and warehouse operator. After the manufacturer produces our medication, it is shipped to a storage facility and stacked in a large transport box. Theft is a heightened risk at any time when goods are changing hands. BSI, the national standards body in the UK, claims that pharmaceutical cargo theft causes an annual loss of 1$ billion every year.
From the warehouse, forwarders bring our medical supply to the airport for overseas delivery. During the journey, special cargo trucks ensure that environmental conditions such as temperature and humidity are maintained within defined parameters for the medicines in question. Even though some companies already use sensors for real-time monitoring, temperature data is often only controlled retrospectively. As a result, drugs frequently suffer damage from mishandling.
According to Biopharmaceutical Reporter, a news outlet specialised in the biopharmaceutical industry, up to 10% of vaccines are lost in transit due to breakage or problems with cold chain infrastructure.
Once they have entered the system, faulty or damaged items are difficult to track down. Such substandard products are not only potentially dangerous to the health of patients, but also cause tremendous waste: the pharmaceutical industry discards at least $15 billion of product each year due to temperature deviations and this sum reaches $35 billion if we take into account additional costs like product replacement.
The risk appears to be disproportionately distributed: for instance, most pharmaceutical manufacturers carry the risk of loss from the point at which they receive raw materials until the finished vaccine is delivered to the distributor. Settling such claims is typically a bureaucratic process, while payouts are far from immediate. Generally, international payments throughout the supply chain are also ridden with hidden costs.
Pain in the chain
After our package arrives at the airport, it is time for customs control. Unfortunately, as a Harvard Business Review analysis highlighted, documentation is often manual and paper-based. As a result, paperwork piles up at each handoff and border crossing. Fragmentation of data across documents and siloed IT systems causes not only delays, but can lead to further errors. Regulations for transportation also differ from country to country, further aggravating the administrative burden, not to mention the looming risk of a fine.
When our medicine finally arrives at the wholesaler or pharmacy, one hurdle remains before it can be placed on store shelves. How can a distributor reliably check if the box of medicines they received is authentic? The question of provenance might seem trivial, but it is a significant challenge. According to Tegan Keele, US blockchain program lead at KPMG, drugs can be traced if they are packed in a box with a barcode or QR code printed on it.
However, visibility decreases as soon as someone opens and unloads that box, because individual units are not yet traceable. This is a major problem: according to a Deloitte report, the pharmaceutical industry loses a massive $200 billion a year due to counterfeiting. However, the gravest cost of counterfeit drugs is their toll in human lives: according to the same report, fake drugs are accountable for about one million deaths per year, a tragedy that particularly affects developing countries.
Blockchain: always on the verge?
As you can see above, the pharmaceutical supply chain is ridden with fragmentation of processes and information asymmetries that make compliance with regulations and the monitoring of sensitive cargo a complex and expensive task. In short, it is an industry with multiple stakeholders with potentially diverging interests, where trust is paramount.
If you are familiar with blockchain technology, this probably sounds like an ideal use case. After all, one of the apparent core purposes of blockchain is to create a trustworthy, secure and inalterable ledger of records accessible to multiple stakeholders. Proponents contend that if this were combined with smart contracts and Internet of Things (IoT) sensors to measure things like temperature, geolocation humidity and shocks, the entire supply chain could be made more transparent. In turn, this would lead to greater accountability and ultimately, less waste. You might even envisage a scenario where paper-based agreements like insurance policies and vendor contracts, and even payments are fully automated.
While this vision sounds alluring, however, unfortunately the current reality is far more modest. In the real world, adoption of blockchain for supply chain management has been rather slow and mainly limited to pilot projects. So why does blockchain seem to be always on the verge but never breaking through to adoption?
One of the core issues is scalability. Managing a global supply chain potentially involves thousands of interactions between individuals and machines. Existing blockchains that rely on proof-of-work (PoW) mining can generally only process about 7 transactions per second on average. Indeed, scalability and excessive energy consumption are frequently cited by researchers as significant barriers to blockchain adoption in supply chain networks, both of which stem directly from PoW mining.
An even greater concern, however, is the technical complexity of blockchain and the degree to which it is interoperable with existing systems. Enterprises tend to want to combine blockchain with their existing back-end systems rather than starting from scratch, but this can often be an onerous undertaking involving high research and development costs. These issues are likely to be particularly prevalent in the pharmaceutical sector, due to the comparative complexity of its supply networks.
Perhaps the most interesting finding across recent studies is that blockchain may be regarded as a potential hindrance rather than a useful tool for solving pharmaceutical supply chain problems. This may be partially attributed to the fact that stakeholders are not comfortable sharing potentially sensitive information with other parties. There is certainly a debate to be had about which information should be kept on and off chain as this is important not only for privacy reasons, but also to ensure that the network does not suffer from excessive bloat.
Geeq: reengineering blockchain
When we were designing Geeq, we knew that if blockchain is to be a viable option for supply chain businesses, it needs to be flexible, upgradable, environmentally sustainable and cost effective.
For this reason, we conceived a new type of consensus mechanism called Proof of Honesty, that does not require costly mining. Unlike other public blockchains, there are no “block proposers” that win the right to validate the next block of transactions. Unlike other enterprise blockchains, Geeq does not delegate the validation of transactions to special, trusted nodes. Instead, all nodes are treated equally, follow the same rules and compete in terms of the accuracy of their ledgers.
By making ledger accuracy, rather than mining power, the fulcrum of competition, we can create blockchains that support far higher transaction throughput, even with modest numbers of nodes. This degree of scalability will be an essential prerequisite for widespread deployment of blockchain in the supply chain sector.
In addition, Geeq has been built from the ground up to be highly flexible, employing a multi-chain architecture that enables developers to design bespoke blockchains for specific business needs. Another facet of this flexibility is that IoT support has been built-in to its core architecture. Data from IoT sensors can be fed directly into a Geeq blockchain network for validation without the need for smart contracts, reducing the complexity of the system and the number of potential attack surfaces for intruders. Indeed, security is where Geeq truly excels, exhibiting both 99% Byzantine fault tolerance and strategically provable security. These safeguards are crucial to create decentralized networks that will be fit for purpose in the coming age of quantum computing.
Conclusion
The pharmaceutical industry could clearly benefit from blockchain-based networks that would enable information and resources to flow through the supply chain with less friction and waste. While blockchain holds the potential to ameliorate these issues, the theory remains far removed from practical reality. At Geeq, we are developing a technology to close this gap. We have designed a new type of blockchain network that can truly enable stakeholders to reduce information asymmetries and coordination problems, while increasing transparency.
A version of this article was published in Asia Crypto Today on March 25, 2021.






To learn more about Geeq, follow us and join the conversation.
@GeeqOfficial